Clinical biological samples analysis
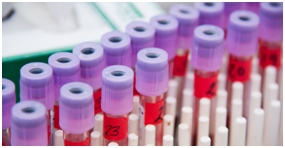
Biological sample analysis services in accordance with CFDA and OECD GLP requirements could be provided, including analytical method development and validation for biological samples. Biological samples from Phase I, II and III clinical trials, clinical bioequivalence test (BE) could be analyzed.
Service items
Biological sample analysis services in accordance with CFDA and OECD GLP requirements could be provided, including:
analytical method development and validation for biological samples;
Biological samples analysis from Phase I, II and III clinical trials;
Biological samples analysis from clinical bioequivalence test (BE);
Compliance guidelines
Good laboratory practice for Non-clinical drug research;
Relevant guidelines published by CFDA CDE;
Instrument and equipment
AB API 4000 Qtrap (LC-MS)、AB API 5500 Qtrap (LC-MS)����、Thermo Finnigan (GC-MS)���、Agilent 1200 (HPLC)����、Waters (UPLC)、Agilent 6890 (GC).
Waters SDMS LIMS; 24-hour temperature monitoring system; Standardized sample management and archives management system; Perfect SOP management system;
Business consulting
Ms.Li +86 024-85869185/ 18540366476